Get the latest news, application notes, and upcoming webinars from XGen Bio Inc.
- About
- About XGen
- Our team
- Follow Us
- Youtube

CONTACT US
Copyright© 2025, Xgen. All rights Reserved.
Terms & Conditions | Privacy Policy | Cookie Policy

INano™ L+
Rapid Nanomedicine Preparation System

INano™ Optimux
Medium-Scale Formulation (Reusable System & GMP Compliant)

INano™ S
Commercial LNP Manufacturing System

XNano™ HT-Smart
End-to-End High-Throughput mRNA/LNP Screening Workstation

XNano™ PCV
LNP Manufacturing System For Emerging Applications
mRNA-LNP kit
Cell Transfection Kit
Application Kit
Organ-specific Targeting Kit
Validation Kit
DNA/Protein-LNP Kit
DNA-LNP Kit
Protein-LNP Kit
One-Stop Shop
LNP Packaging
Request a Quote Get Brochure
XGen proudly offers lipid nanoparticle (LNP) formulation services for mRNA and circular RNA drug research and development service.
We are excited to expand our offerings with the introduction of our new Targeted LNP Service. This advanced service is engineered to enhance the precision and efficacy of therapeutic delivery by utilizing state-of-the-art lipid formulations and targeting ligands. Our Targeted LNP Service ensures that therapeutic payloads are delivered directly to specific cells or tissues, thereby maximizing therapeutic impact and minimizing off-target effects.
LNPs have been proven to be an effective drug delivery system for RNA therapeutics which can be easily degraded in vivo. The lipid layer can fuse with the cell membrane, allowing the encapsulated RNA to enter the cell and exert its therapeutic effect. They also protect therapeutic agents from degradation and improve their bioavailability LNPs are used in various applications, such as delivering RNA-based therapeutics or small molecules.
 |
 |
 |
||
| Variety of ionizable lipid formulation with high encapsulation efficiency | Variety of ionizable lipid formulation with high encapsulation efficiency | Ready-Edit LNP Formulation with CRISPR RNA solutions |
| LNP Service Options | ||||||||||
| Characteristics | QC Testing | Payload Requirement | Application | |||||||
|
|
|
|
|||||||
| Manufacturing Workflow | |||||||||
| Our integrated IVT RNA manufacturing workflow streamlines production from gene synthesis to LNP packaging. | |||||||||
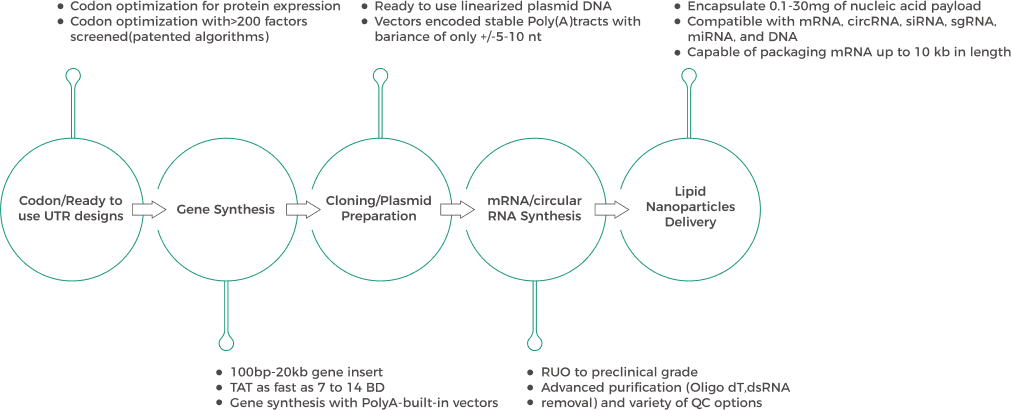 |
|||||||||
| Quality Control and Specification | |||||||||
| Project | Detection | Testing Standards | Standard QC | Advanced QC | |||||
| Appearance | Visual observation | Clear and free of foreign particles | √ | √ | |||||
| Final Concentration | RiboGreen assay | 0.05 - 0.2 mg/mL | √ | √ | |||||
| Encapsulation Efficiency | RiboGreen assay | >85% | √ | √ | |||||
| Encapsulated RNA Integrity | Bioanalyzer | >75% | × | √ | |||||
| Partical Size | DLS |
MC3-LNP 80 - 110 nm SM102-LNP 65 - 125 nm Lipid 5-LNP 65 - 125 nm ALC0315-LNP 50 - 100 nm |
√ | √ | |||||
| PDI | DLS | <0.2 | √ | √ | |||||
| Zeta Potential | DLS | ± 15.0 mV | × | √ | |||||
| pH | pH meter | 7.4 ± 0.5 | × | √ | |||||
| Endotoxin | LAL endotoxin detection | <10 EU/mL | × | √ | |||||
| Bioburden | Pour plate and spread plate assay | <10 CFU/100mL | × | √ | |||||