Get the latest news, application notes, and upcoming webinars from XGen Bio Inc.
- About
- About XGen
- Our team
- Follow Us
- Youtube

CONTACT US
Copyright© 2025, Xgen. All rights Reserved.
Terms & Conditions | Privacy Policy | Cookie Policy

INano™ L+
Rapid Nanomedicine Preparation System

INano™ Optimux
Medium-Scale Formulation (Reusable System & GMP Compliant)

INano™ S
Commercial LNP Manufacturing System

XNano™ HT-Smart
End-to-End High-Throughput mRNA/LNP Screening Workstation

XNano™ PCV
LNP Manufacturing System For Emerging Applications
mRNA-LNP kit
Cell Transfection Kit
Application Kit
Organ-specific Targeting Kit
Validation Kit
DNA/Protein-LNP Kit
DNA-LNP Kit
Protein-LNP Kit
INano™ S
Commercial LNP Manufacturing System
Request a Quote Get Brochure

INanoTM S is a GMP-compliant commercial-scale production equipment that integrates upstream and downstream processes, including LNP encapsulation and TFF. This instrument has been successfully utilized for commercial-scale production of COVID vaccines, demonstrating its reliability and efficiency in meeting high-demand manufacturing requirements.
Flowrate range: 1-4 L/min (without dilution)
Designed with the QbD (Quality by Design) concept, allowing full adjustability of key process parameters
Compatible with public utilities such as water-for-injection and high-temperature steam for CIP/SIP, with a robust design to minimize contamination risks
Offers flexibility to use microfluidic mixing chips or T-mix mixers without the need for additional consumables.
Proven success in multiple commercial-scale production for LNPs.
|
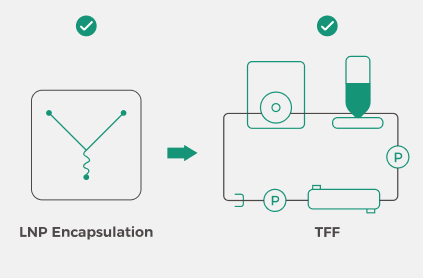
Integration of LNP Encapsulation and Downstream Processing
The Inano™ S is large-scale commercial GMP production equipment. It innovatively integrates LNP encapsulation with downstream TFF purification and concentration processes, achieving full process continuity and automation. |
 |
|
|
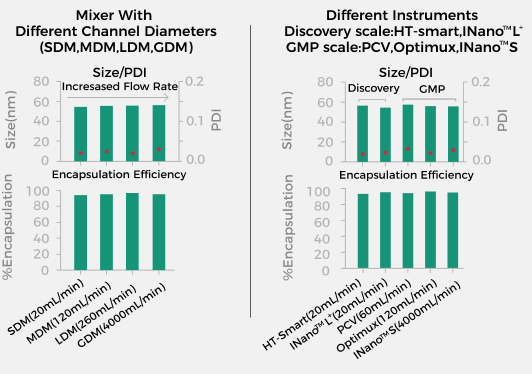
Multiple Mixing Methods
As a platform-based device, the INano™ S not only supports microfluidic mixer and T-mixer structures, but also allows for the customization of mixing structures based on customer requirements. Additionally, it offers a variety of mixer materials for users to choose from, such as 316L stainless steel, Hastelloy, and more. |
Ensure that all preparation process parameters remain unchanged to avoid secondary process development, guaranteeing a high success rate for scale-up, allowing you to easily become a process expert! |
 |
|
GMP Complicance
The equipment is meticulously designed and manufactured to comply with GMP, cGMP, and EudraLex regulations. Adherence to ASME and BPE standards ensures precision and quality throughout the process. Constructed with premium materials and components, the equipment delivers exceptional stability and reliability, meeting the highest industry standards. |
 |
|
|
Comprehensive Documentation and Full Traceability
The equipment's operating system is designed in strict compliance with FDA 21 CFR Part 11, incorporating multi-level user access management, data recording, audit trails, electronic signatures, and may more additional features. |
