Get the latest news, application notes, and upcoming webinars from XGen Bio Inc.
- About
- About XGen
- Our team
- Follow Us
- Youtube

CONTACT US
Copyright© 2025, Xgen. All rights Reserved.
Terms & Conditions | Privacy Policy | Cookie Policy

INano™ L+
Rapid Nanomedicine Preparation System

INano™ Optimux
Medium-Scale Formulation (Reusable System & GMP Compliant)

INano™ S
Commercial LNP Manufacturing System

XNano™ HT-Smart
End-to-End High-Throughput mRNA/LNP Screening Workstation

XNano™ PCV
LNP Manufacturing System For Emerging Applications
mRNA-LNP kit
Cell Transfection Kit
Application Kit
Organ-specific Targeting Kit
Validation Kit
DNA/Protein-LNP Kit
DNA-LNP Kit
Protein-LNP Kit
INano™ Optimux
Medium-Scale Formulation (Reusable System & GMP Compliant)
Request a Quote Get Brochure

INano™ Optimux is a highly versatile, GMP-compliant, multifunctional continuous nanomedicine preparation system. It supports automated process development for scaling up nanomedicines like LNPs and facilitates GMP production of clinical-stage products. Whether it’s large-scale process development for early CMC studies or upstream or downstream process research, this system can do it all with unparalleled flexibility and reliability.
|
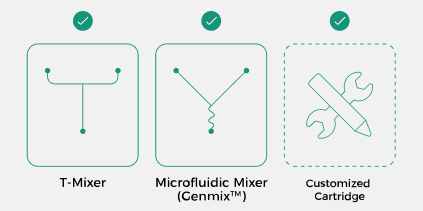
Multiple Mixing Methods
As a platform device, the INano™ Optimux not only supports microfluidic/T-mixer cartridges but also allows for custom cartridge designs based on customer requirements and offers a variety of cartridge materials to choose from. |
 |
|
|
Intelligent Process Screening
INano™ Optimux is equipped with an automated clean in-place (CIP) system, an automated sample collection system, and an automated perfusion system. Users can perform automated screening based on different process conditions during process development, significantly accelerating development speeds. |
|
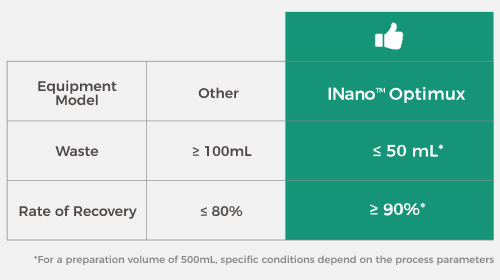
Flexibly Adapt to Different Production Scales
INano™ Optimux supports micro-volume preparation with a minimum of 300 μL and GMP production up to 100 L. Its unique patented control algorithm can limit waste liquid volume to ≤ 50 mL*. |
 |
|
|
Powerful Automatic CIP System
Traditional LNP preparation equipment requires manual cleaning and endotoxin control, with frequent changes of cleaning solutions. This process is tedious and lacks system recording, posing significant compliance challenges. The INano™ Optimux integrates an automatic CIP system, enabling a truly hands-free, fully automated process with complete system documentation, allowing you to say goodbye to the dilemma of "3 hours of cleaning, 10 minutes of preparation"! |
|
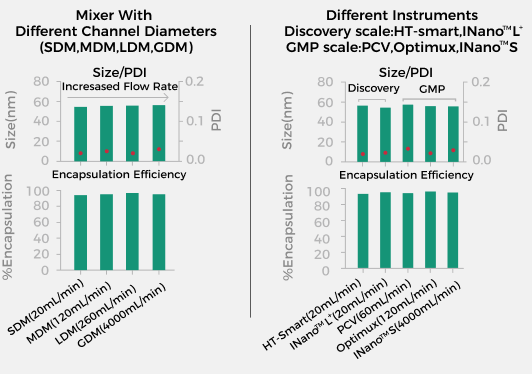
Consistent Critical Quality Attributes (CQA) Can Be Achieved From Preclinical to Clinical Development
INano™ Optimux adopts the same scalable mixing structure as INano L+, ensuring the consistency of critical quality attributes (CQA) throughout the development process and significantly accelerating the technical transfer from process development to GMP production. |
 |
|
|
Regulatory Compliance
The equipment is meticulously designed and manufactured to comply with GMP, cGMP, and EudraLex regulations. Adherence to ASME and BPE standards ensures precision and quality throughout the process. Constructed with premium materials and components, the equipment delivers exceptional stability and reliability, meeting the highest industry standards. |
|
Complete documentation and traceability
The equipment operating system is designed in strict compliance with FDA 21 CFR Part 11, featuring multi-level user access management, data-only recording, audit trails, electronic signatures, and more. |
 |
