Get the latest news, application notes, and upcoming webinars from XGen Bio Inc.
- About
- About XGen
- Our team
- Follow Us
- Youtube

CONTACT US
Copyright© 2025, Xgen. All rights Reserved.
Terms & Conditions | Privacy Policy | Cookie Policy

INano™ L+
Rapid Nanomedicine Preparation System

INano™ Optimux
Medium-Scale Formulation (Reusable System & GMP Compliant)

INano™ S
Commercial LNP Manufacturing System

XNano™ HT-Smart
End-to-End High-Throughput mRNA/LNP Screening Workstation

XNano™ PCV
LNP Manufacturing System For Emerging Applications
mRNA-LNP kit
Cell Transfection Kit
Application Kit
Organ-specific Targeting Kit
Validation Kit
DNA/Protein-LNP Kit
DNA-LNP Kit
Protein-LNP Kit
XNano™ PCV
LNP Manufacturing System For Emerging Applications
Request a Quote Get Brochure

XNano™ PCV is the world’s first fully enclosed, single-use LNP encapsulation system designed for emerging applications such as personalized cancer vaccines, in vivo CAR-T therapies, and more. It enables efficient production scaling from 40 mL to 4.5 L.
System setup is simple and can be completed in just 5 minutes
Preparation volume ranges from 40 mL to 4,500 mL
Designed with the QbD (Quality by Design) concept in mind, critical process parameters are adjustable
Four-channel cartridge design accommodates a wider range of processes
Highly accurate flow rate control with real-time monitoring via an integrated flow rate sensor
End-to-end system reduces the risk of contamination during both experimental and production processes
Single-use fluid path eliminates the need for complex cleaning validation
|
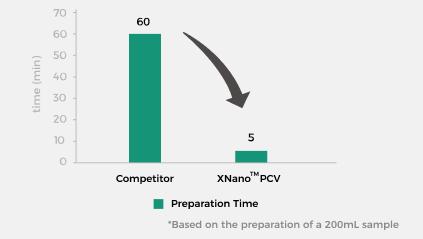
Simple and Efficient, No Cleaning Validation Required
The XNano™ PCV simplifies the process by eliminating complex steps such as system cleaning. From assembly to formulation completion, the entire process takes just 5 minutes*. |
 |
|
|
Innovative and Future-Focused
The patented four-channel cartridge design enables multiple dilution processes while accommodating the requirements of differentiated delivery systems. It expands application possibilities, including antibody-LNP conjugation and core-shell delivery systems. |
|
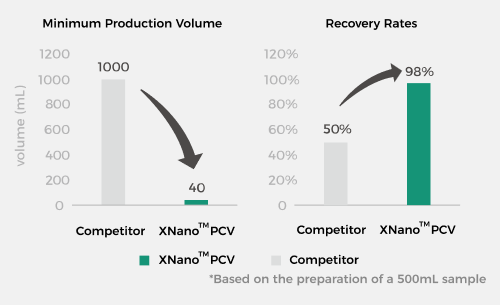
Cost Reduction and Efficiency Enhancement, Making Drugs Accessible
XGen's patented microfluidic technology and processes enable a minimum preparation volume of 40 mL and a sample recovery rate as high as 98%*, making drugs more accessible than ever. |
 |
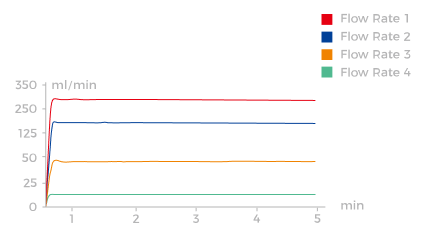 |
Intelligently Manufactured with 'Core' Precision
The I-Control and I-Sensor algorithms precisely control and display the real-time flow rate with millisecond-level accuracy. |
|
GMP Complicance The equipment is meticulously designed and manufactured to comply with GMP, cGMP, and EudraLex regulations. Adherence to ASME and BPE standards ensures precision and quality throughout the process. Constructed with premium materials and components, the equipment delivers exceptional stability and reliability, meeting the highest industry standards. |
 |
|
|
Comprehensive Documentation and Full Traceability
The equipment's operating system is designed in strict compliance with FDA 21 CFR Part 11, incorporating multi-level user access management, data recording, audit trails, electronic signatures, and may more additional features. |
